DIVISION ll—HEALTH PROVISIONS TITLE I—PUBLIC HEALTH Subtitle A—National Disaster Medical System Subtitle B—Synthetic Nic
Referencing the Definition of “Device” in the Federal Food, Drug, and Cosmetic Act in Guidance, Regulatory Documents, Commun
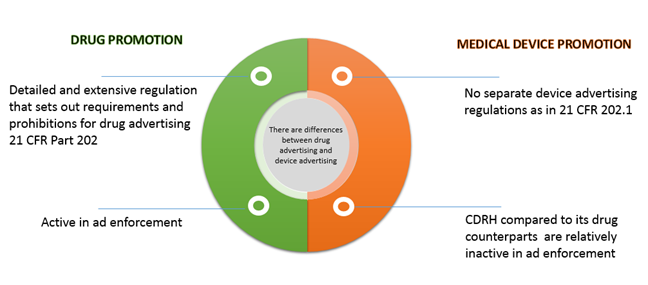
Is my Product a Medical Device? Slide 1 Hello, my name is Commander Kimberly Piermatteo of the United States Public Health Servi
that the repayment charge exceeds the amount of positiye direct investment so authorized in such scheduled area, fur ther redu
PUBLIC LAW 86-618-JULY 12, 1960 397 Public Law 86-618 Be it enacted hy the Senate and House of Representatives of the United Sta